Chem 1220 Supplemental Recitation Activity Week 4
化学作业代写 Potassium chloride (Molar mass = 74.55 g/mol) has a solubility of 32 g per 100 g of water at 20°C, where the density of water is 0.998 g/mL.
1.
The enthalpy change associated with forming a solution ΔHsoln is given by the following equation:
ΔHsoln = ΔHsolute + ΔHsolvent + ΔHmix
Which of the four enthalpy terms is always exothermic?
(a) ΔHsolute
(b) ΔHsolvent
(c) ΔHmix
(d) ΔHsoln
(e) None of the above
2. 化学作业代写
Which of the following substances would you expect to be the least soluble in water?
(a) Methanol, CH3OH
(b) Hexanol, CH3CH2CH2CH2CH2CH2OH
(c) Acetone, CH3COCH3
(d) Ammonium nitrate, NH4NO3
3.
Potassium chloride (Molar mass = 74.55 g/mol) has a solubility of 32 g per 100 g of water at 20°C, where the density of water is 0.998 g/mL. What is the molarity of a saturated solution of potassium chloride?
(a) 0.043 M
(b) 0.24 M
(c) 2.4 M
(d) 4.3 M
(e) 32 M
4. 化学作业代写
Calculate the freezing point of a solution containing 0.600 kg of chloroform (CHCl3, molar mass = 50.5 g/mol, freezing point = −63.5 °C, Kf = 4.68 °C/m) and 42.0 g of eucalyptol (C10H18O, molar mass = 154.2 g/mol, freezing point = 1.5 °C), a fragrant substance found in the leaves of eucalyptus trees.
(a) −2.12 °C
(b) −61.4 °C
(c) −63.9 °C
(d) −65.6 °C
(e) −67.1 °C
5.
An aqueous solution of acetone (C3H6O, molar mass = 58.1 g/mol) has a concentration of 7.25 M and a density of 0.925 g/mL. What is the mole fraction of acetone in this solution?
(a) 0.073
(b) 0.116
(c) 0.206
(d) 0.455
(e) 14.4
6. 化学作业代写
If the vapor pressure of pure acetone and water at 25 °C are 230 torr and 23.8 torr, respectively, what is the mole fraction of acetone in the vapor that is in equilibrium with the solution from the previous problem?
(a) 0.145
(b) 0.206
(c) 0.474
(d) 0.715
(e) 0.906
7.
Adrenaline is the hormone that triggers the release of extra glucose molecules in times of stress or emergency. A solution containing 0.640 g adrenaline and 36.0 g CCl4 elevates the boiling point of carbon tetrachloride by 0.49 °C. If the boiling point elevation constant for CCl4 Kb= 5.02 °C/m what is the approximate molar mass of adrenaline?
(a) 97.6 g/mol
(b) 182 g/mol
(c) 275 g/mol
(d) 351 g/mol
(e) 548 g/mol
8. 化学作业代写
What is the typical size range for the dispersed substance in a colloidal dispersion?
(a) 1 Å to 100 Å
(b) 1 nm to 100 nm
(c) 5 nm to 1 μm
(d) 1 μm to 5 μm
9.
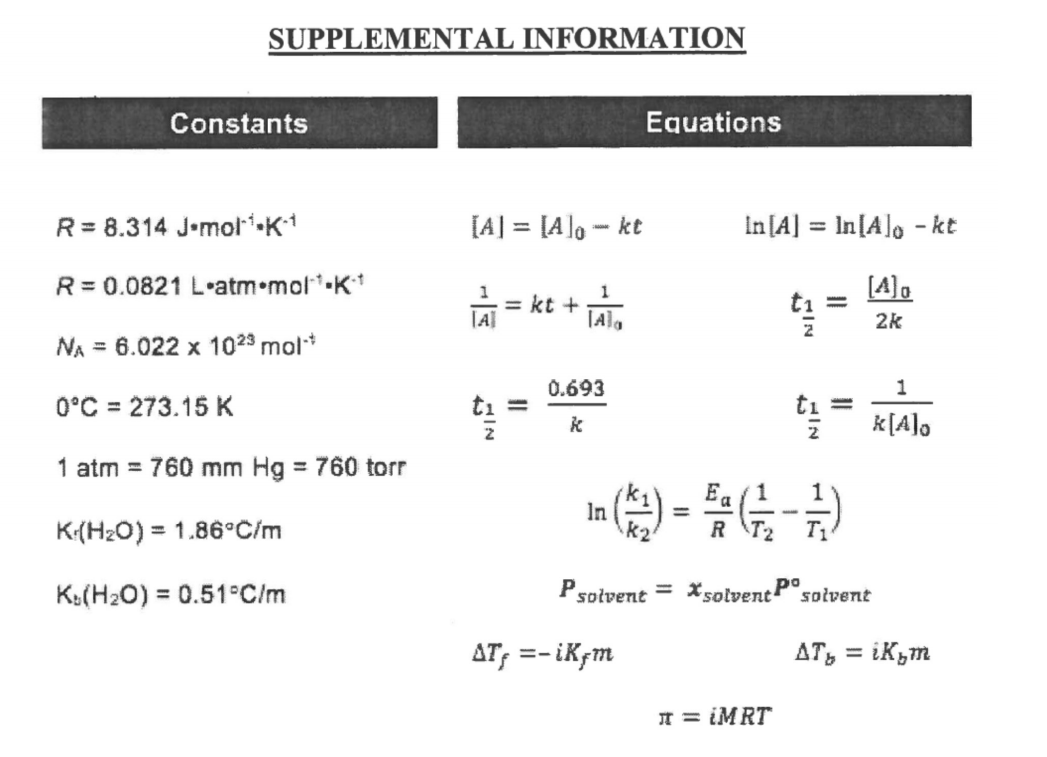
Only one of the plots below only one is consistent for the second order reaction A → B. Circle the correct plot.
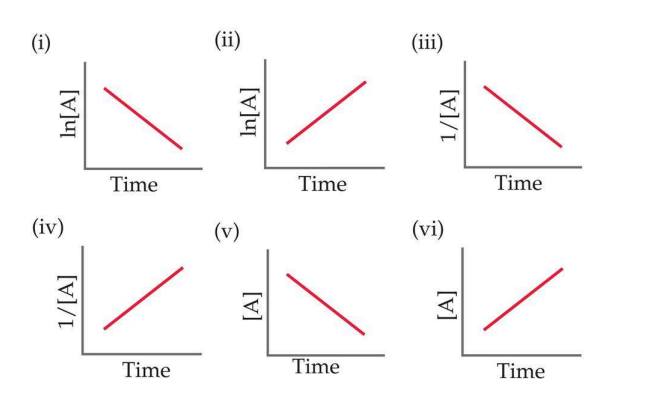
10.
The following data were collected for the rate of disappearance of NO in the reaction: 2NO(g) + O2(g) 2NO2(g)
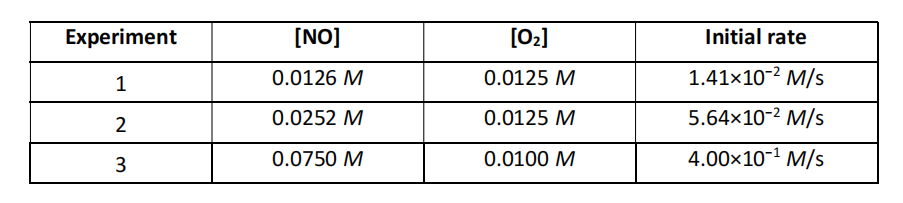
What is the rate law and rate constant for this reaction?
(a) Rate = k [NO][O2] with k = 89.5 M-1 s-1
(b) Rate = k [NO]2[O2] with k = 7.10×103 M-2 s-1
(c) Rate = k [NO][O2]2 with 7.16×103 M-2 s-1
(d) Rate = k [NO][O2] with k = 14.1 M-1 s-1
(e) Rate = k [NO]2 [O2] with k = 89.5 M-2 s-1
11.
What is the initial rate of disappearance of NO ions in experiment 2 of the previous problem?
(a) 2.82×10-2 M/s
(b) 5.64×10-2 M/s
(c) 0.113 M/s
(d) 2.52×10-2 M/s
(e) None of the above
12. 化学作业代写
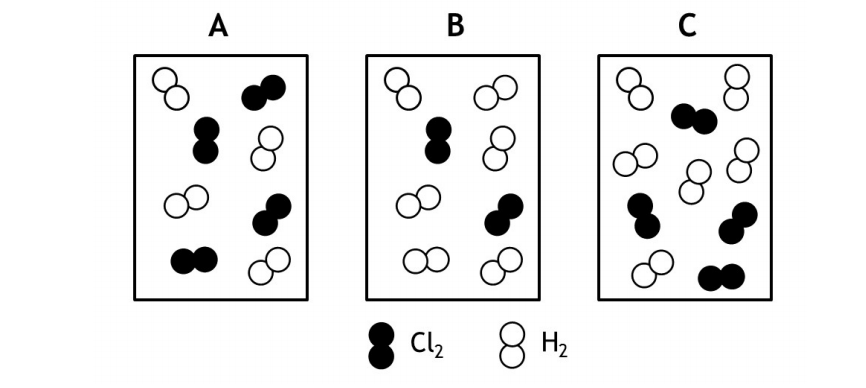
The rate law for the reaction Cl2(g) + H2(g) → 2HCl(g) is determined to be first order in Cl2 and zeroth order in H2. Given that information rank order the following mixtures from the fastest to the slowest initial rate of reaction.
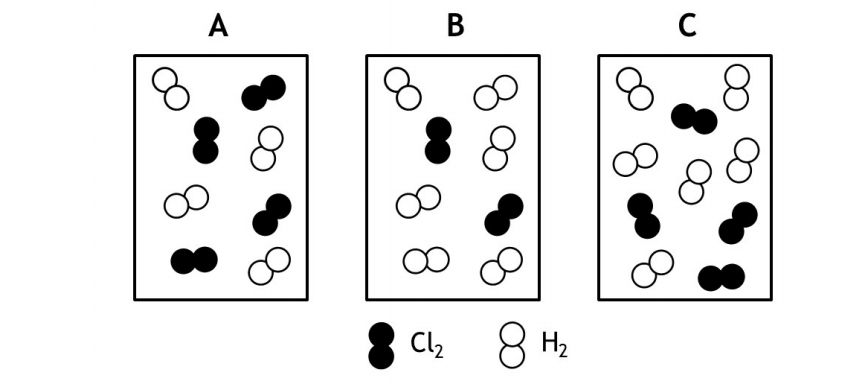
(a) Fastest B = C > A Slowest
(b) Fastest B > A > C Slowest
(c) Fastest A = C > B Slowest
(d) Fastest B > C = A Slowest
(e) Fastest C > B = A Slowest
13.
The reaction 2NO2(g) → 2NO(g) + O2(g) is second order in NO2 with a rate constant k = 0.542M-1 s-1. If the initial concentration of NO2 in a closed vessel is 0.0500 M what is the concentration of NO2 after 30.0 minutes?
(a) 9.85 × 10-4 M
(b) 1.00 × 10-3 M
(c) 1.76 × 10-3 M
(d) 7.85 × 10-3 M
(e) 2.76 × 10-3 M
14. 化学作业代写
For the elementary reaction Cl(g) + HBr(g) → HCl(g) + Br(g) the overall energy change associated with the reaction is −66 kJ and the activation energy is 7 kJ. What is the activation on energy of the reverse reaction?
(a) −7 kJ
(b) 7 kJ
(c) 59 kJ
(d) 66 kJ
(e) 73 kJ

更多代写:代写PHP 代考推荐 英国公共关系论文写作 博士Essay代写 dissertation代寫价格 微积分网课代修