General Chemistry Lecture I (Chem 001-01) Exam 3
Monday, November 18, 2019
9:00-9:50 Reiss 103
Chemistry化学代考 Estimate the contribution of motion to the molar internal energy of ethene gas, C2H4, at 25 ° Ignorevibrations.
Please show all your work.
Try to make your flow of logic neat and obvious. There are a total of 100 points in this exam.
Name (please print):
My signature signifies that I have neither given nor received help on this exam. Chemistry化学代考
Honor Pledge (please sign):
| Problem | Points | Score |
| 1 | 2.5 | |
| 2 | 7.5 | |
| 3 | 8 | |
| 4 | 20 | |
| 5 | 12 Chemistry化学代考 | |
| 6 | 10 | |
| 7 | 15 | |
| 8 | 15 | |
| 9 | 10 | |
| Total | 100 |
R = 0.0821 L atm/mol K = 8.314 J/mol K
°C + 273.15 = K 1 atm = 760 mm Hg
101.325 J = 1 L atm NA = 6.022 x 1023 PV = nRT ΔU = q + w
ΔH = ΔU + PV = ΔU + ΔnRT
w = -Pext ΔV w = -nRT ln(V2/V1) Chemistry化学代考
q = CΔT Σ heat changes = 0
CP,m = CV,m + R
ΔHºrxn = Σ n ΔHfºproducts – Σ n ΔHfºreactants
for ideal gas atoms CV,m = 3/2 R
for ideal gas linear molecules CV,m = 5/2 R for ideal gas nonlinear molecules CV,m = 3 R
ΔCP = Σ n ΔCP,m products – Σ n ΔCP,m reactants ΔHº(T2) = ΔHº(T1) + (T2 – T1) ΔCP Density of a unit cell = [(molar mass)(1/NA)(#lattice points/unit cell)]/(length of edge)3 ΔHºrxn = Σ Bond enthalpies of bonds broken – Σ Bond Enthalpies of bonds formed
1.If a solid line represents a covalent bond and a dotted line represents intermolecularattraction, which of these choices shows a hydrogen bond?
Circle all that apply. (2.5 points)
a) —N·······H—N—
b) O—H·······H—C c)
—O·······H—O— d)
—F·······H—F— e)
—O·······H—C—
2.a) Of the followingsubstances, only has London dispersion forces as the only intermolecular force.
A)CH3OH
B)NH3
C)H2S
D)Kr
E)HCl
b)Which compound has the highest boilingpoint? Chemistry化学代考
A)CBr4 (carbontetrabromide)
B)C12H26
C)CI4 (carbontetraiodide)
D)N2
E)O2
c)Which of the following molecules would deviate from ideal gas behavior the most at 298K?
A)Ne
B)O2
C)H2O
D)CO
E)CH4
3.a) What is the coordination number of iron if the metal has a body-centered cubic unitcell? Chemistry化学代考
b)What is the coordination number of Ne when it is crystallized in a face-centered cubic unitcell?
c)What is the coordination number in a primitive cubiclattice?
d)What is the coordination number of sodium ions in the NaCl structure (i.e. how many chlorides surround each sodiumion)?
- (20points)
a)The density of solid krypton is 2.16 g/cm3. Krypton crystallizes in a cubic close-packed structure; estimate the atomic radius of Kr.
b)CsCl crystallizes in a unit cell that contains the Cs+ion at the center of a cube that has a Cl- at each corner. Each unit cell contains Cs+ ion(s) and Cl-, ion(s). Chemistry化学代考
c)CsCl crystalizes in a cubic unit as described in part b. If the length of the edge of the unit cellis 412.3 pm, what is the approximate Cs-Cl bond length?
- (12points)
a)Crystalline solids differ from amorphous solids in that crystallinesolids have .
A)appreciable intermolecular attractiveforces
B)a long-range repeating pattern of atoms, molecules, orions
C)atoms, molecules, or ions that are closetogether
D)much larger atoms, molecules, orions
E)no orderlystructure
b) solids consist of atoms or molecules held together by dipole-dipole forces, London dispersion forces, and/or hydrogen bonds. Chemistry化学代考
A)Ionic
B)Molecular
C)Metallic
D)Covalent-network
E)Metallic andcovalent-network
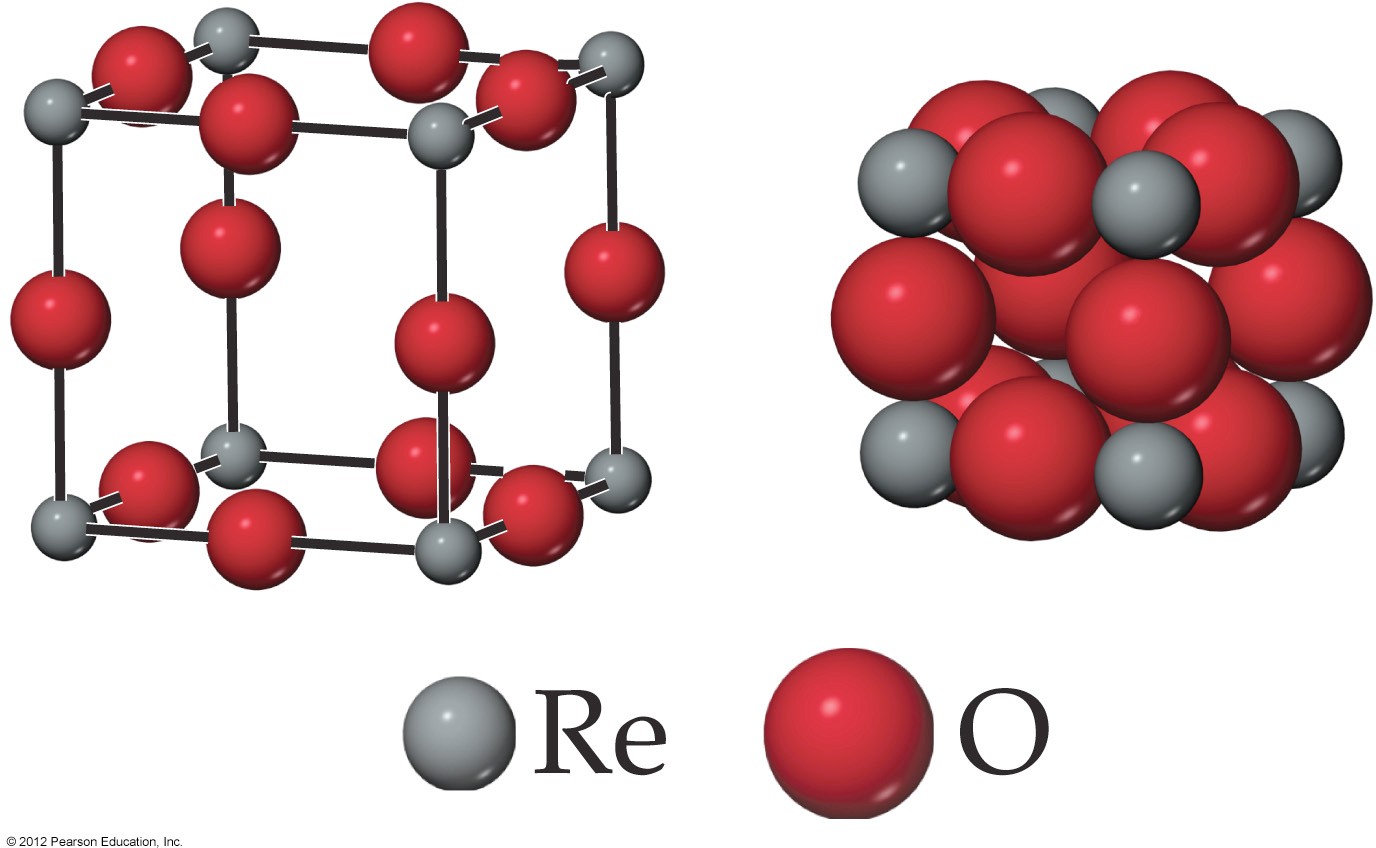
c)A cubic unit cell of a compound containing only Re and O, contains rhenium ions at the corners and oxide ions at the center of each edge as shown below. What is the empirical formula of the oxide? Show your work to prove your answer. Just the answer will not earncredit.
6.(10points) Chemistry化学代考
How much energy is required to convert a 18.0 g ice cube at -19.0 °C to water vapor at 100.0 °C?
specific heat of ice = 2.09 J/g K
specific heat of liquid water = 4.184 J/g K specific heat of steam = 1.84 J/g K
the enthalpy of fusion of water = 6.01 kJ/mol
the enthalpy of vaporization of water is 40.67 kJ/mol
- (15points)
a)In a combustion chamber, the total internal energy change produced from burning a fuel is -2573 kJ. The cooling system that surrounds the chamber absorbs 947 kJ as heat. How much work can be done by the fuel in thechamber?
b)Calculate the work, in units of J, for each of the following processes beginning with a gas sample in a piston assembly with T = 305K, P = 1.79 atm and V =29L:
i)irreversible expansion against a constant external pressure of 1.00 atm to a final volume of 6.52L Chemistry化学代考
ii)isothermal, reversible expansion to a final volume of 6.52L
c)Estimate the contribution of motion to the molar internal energy of ethene gas, C2H4, at 25 ° Ignorevibrations.
8.(15points) Chemistry化学代考
a) Calculate the standard enthalpy of formation of N2O5(g)from the following data: 2 NO(g) + O2 (g) ® 2 NO2 (g) ΔH° = -114.1 kJ
4 NO2 (g) + O2 (g) ® 2 N2O5 (g) ΔH° = -110.2 kJ
½ N2 (g) + ½ O2 (g) ® NO(g) ΔH° = 90.3 kJ
- (10points)
Calculate the lattice enthalpy of potassium fluoride from the following data.
enthalpy of formation of K(g): +89 kJ/mol
first ionization energy of K(g): +418 kJ/mol enthalpy of formation of F(g): +79 kJ/mol
electron affinity of F(g): +328 (ΔH = -328) kJ/mol enthalpy of formation of KF(s): -567 kJ/mol
The Periodic Table of the Elements
1995 IUPAC masses and Approved Names from http://www.chem.qmw.ac.uk/iupac/AtWt/ masses for 107-111 from C&EN, March 13, 1995, p. 35 112 from http://www.gsi.de/z112e.html

更多代写:c++作业代写 生物学考试代考 生物专业essay代写 实验Essay代写 市场学论文代写 留学生计算机论文代写
