Benzene
Name
Institution
有机化合物essay代写 It has numerous industrial uses including being a significant constituent of gasoline, a solvent for many processes, a cleaner for major parts
Benzene
Introduction
Benzene is an aromatic hydrocarbon with a pleasant smell and has no color. It is highly flammable, volatile, miscible in organic solvents but slightly soluble in water. It occurs naturally through forest fires or volcanic eruptions, but a significant amount results from human activities and industrial processes. In the United States, it is among the top 20 chemicals commonly used in industries and other purposes. Most of the aromatic substances are derived from benzene and are referred to as benzenoid compounds or benzenoids, for example, ethylbenzene, cumene, and aniline. It is also a natural constituent of cigarette smoke, gasoline and crude oil. Despite its numerous benefits to humans, it also poses a great health danger because it is thought to be carcinogenic causing leukemia and other blood disorders (American Cancer Society, 2013). The symbol of benzene is as shown.
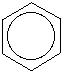
(Singh, 2004).
译文:
苯 有机化合物essay代写
介绍
苯是一种芳香烃,气味宜人,没有颜色。 极易燃,易挥发,易溶于有机溶剂,微溶于水。 它通过森林火灾或火山爆发自然发生,但很大一部分来自人类活动和工业过程。 在美国,它是工业和其他用途常用的前 20 种化学品之一。 大多数芳香族物质来源于苯,被称为苯系化合物或苯系物,例如乙苯、枯烯和苯胺。 它也是香烟烟雾、汽油和原油的天然成分。 尽管它对人类有很多好处,但它也构成了很大的健康危险,因为它被认为是致癌的,会导致白血病和其他血液疾病(美国癌症协会,2013 年)。 苯的符号如图所示。
History of Benzene
Michael Faraday first discovered and isolated benzene in 1825. He discovered an oily liquid that was produced at high pressure in coal tanks that stored illuminating gas. Faraday also discovered that the product had the same number of hydrogen and carbon, so he called it carbureted hydrogen. Laurent was a chemist who had differing thoughts about the naming of the compound. He suggested the name pheno, which means to shine because the compound was derived from a lighting gas.

However, his proposal was not accepted, but the name was carried on to present day as phenyl to represent the C6H5– compounds. In 1835, Eilhardt Mitscherlich heated benzoic acid with lime and obtained the same product as Faraday. He named the compound benzin and identified that the empirical formula of the product was C6H6. Other scientists disagreed with the name benzin because alkaloids bear the same name, which could be misleading. The name benzol was also proposed, but also rejected to avoid confusion with names of alcohols that end with –ol. Thus, the name benzene was adopted because it was used in both France and England (Singh, 2004).
译文:
苯的历史 有机化合物essay代写
迈克尔法拉第于 1825 年首次发现并分离出苯。他发现了一种油状液体,在高压下在储存照明气体的煤罐中产生。法拉第还发现该产品具有相同数量的氢和碳,因此他称其为碳化氢。 Laurent 是一位化学家,他对化合物的命名有着不同的想法。他建议使用pheno这个名字,意思是发光,因为这种化合物来自一种照明气体。
然而,他的提议没有被接受,但该名称沿用至今,作为苯基代表 C6H5-化合物。 1835 年,Eilhardt Mitscherlich 用石灰加热苯甲酸,得到与法拉第相同的产物。他将该化合物命名为苯并确定该产品的经验式为 C6H6。其他科学家不同意苯辛这个名称,因为生物碱具有相同的名称,这可能会产生误导。苯的名称也被提议,但也被拒绝,以避免与以 -ol 结尾的酒精名称混淆。因此,采用苯这个名称是因为它在法国和英国都有使用(Singh,2004)。
Structure of Benzene
Friedrich August Kekule was the first chemist to discover that benzene has a ring structure, in 1865 while Kathleen Lonsdale, a crystallographer, confirmed the cyclic structure. Researchers found out that the carbon-carbon bonds were identical in length, unlike the conventional fact that a double bond is shorter than a single bond. The angle between the bonds is 120°. The bond length of a benzene molecule is also greater than a normal double bond but does not exceed that of a single bond. X-ray diffraction was used to examine and study the structure of the benzene molecule, deducing the aforementioned characteristics of the structure.
译文:
苯的结构 有机化合物essay代写
弗里德里希·奥古斯特·凯库勒 (Friedrich August Kekule) 是第一个发现苯具有环状结构的化学家,1865 年,晶体学家凯瑟琳·朗斯代尔 (Kathleen Lonsdale) 证实了这种环状结构。 研究人员发现,碳-碳键的长度是相同的,这与双键比单键短的传统事实不同。 键之间的角度为 120°。 苯分子的键长也大于正常的双键,但不超过单键的键长。 X射线衍射被用来检查和研究苯分子的结构,推导出上述结构特征。
The structure of benzene constitutes six atoms of carbon to form a planar regular hexagon.
All the atoms in the benzene molecule are on the same plane; thus, the name planar. Covalent bonds join the individual carbon atoms to the other, which have sp2 hybridization. The planar shape occurs due to the sideways overlapping of the p orbitals resulting in a delocalized pi system and electrons. Two atom neighbors surround each carbon atom; thus, it utilizes two electrons from each atom. An additional electron from the carbon atom has the role of bonding the hydrogen atom attached to the carbon.
The other six electrons make right-angled revolution around the nucleus of the atom as they overlap each other.
Subsequently, there is the equal sharing of electrons with three being in the upper ring and three in the lower ring, forming two clouds of electrons below and above the carbon ring plane. Therefore, the six electrons are termed as delocalized because they are not restricted to distinct carbon atoms. The resultant structure is that of a hexagon with a circle in the middle. The resonance theory was deduced to elucidate the structure of benzene and why it is less reactive than alkenes. The theory supposes that hybrid molecules composed of two or more Lewis structures exhibit greater stability than non-hybrid molecules.
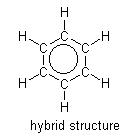
(Stoker, 2011).
Thus, the stability of benzene and other aromatic molecules make them less reactive than alkenes. Delocalization of the electrons also reduces the reactivity of benzene as compared to the alkenes.
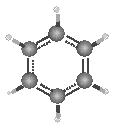
Delocalized electrons with a circular ring at the center (Singh, 2004).
译文:
苯的结构由六个碳原子组成,形成一个平面规则六边形。
苯分子中的所有原子都在同一平面上;因此,名称平面。共价键将单个碳原子连接到另一个,它们具有 sp2 杂化。平面形状的出现是由于 p 轨道的横向重叠导致离域 pi 系统和电子。每个碳原子周围有两个原子邻居;因此,它利用来自每个原子的两个电子。来自碳原子的额外电子具有将氢原子连接到碳上的作用。
其他六个电子在相互重叠时围绕原子核进行直角旋转。
随后,电子均等共享,三个在上环,三个在下环,在碳环平面下方和上方形成两个电子云。因此,六个电子被称为离域电子,因为它们不限于不同的碳原子。由此产生的结构是一个六边形,中间有一个圆圈。共振理论被推导出来阐明苯的结构以及为什么它的反应性低于烯烃。该理论假设由两个或多个路易斯结构组成的杂化分子比非杂化分子表现出更大的稳定性。
因此,苯和其他芳香族分子的稳定性使它们的反应性低于烯烃。与烯烃相比,电子的离域化也降低了苯的反应性。
Manufacturing of Benzene
Benzene is produced through catalytic reforming, which is a process used to produce aromatic compounds from various raw materials. This process accounts for about 30% of commercial benzene globally. The methods of extracting the aromatic compounds include dehydroisomerizing alkyl cyclopentanes, dehydrogenating cycloparaffins, and paraffin dehydrogenation and cyclization. Solvent extraction techniques are used to recover the benzene yielded from the reformate.
Another process through which benzene is produced is the hydrodemethylation of toluene in the presence of a catalyst or heat. The most common catalytic processes include DETOL and Hydeal while thermal processes include HDA and THD. This process contributes to about 25% of the world’s benzene production. In addition, coal tar is also used to produce benzene where the tar acids are removed with caustic soda. Distillation at the lowest boiling fraction is used to yield benzene, which is then purified through hydrodealkylation (Schwartz, 2013).
译文:
苯的制造
苯是通过催化重整生产的,催化重整是一种用于从各种原料生产芳族化合物的过程。这一过程占全球商业苯的 30% 左右。提取芳香族化合物的方法包括烷基环戊烷脱氢异构、环烷烃脱氢、石蜡脱氢环化等。溶剂萃取技术用于回收重整产品中产生的苯。
另一种生产苯的过程是在催化剂或热量存在下甲苯的加氢脱甲基。最常见的催化过程包括 DETOL 和 Hydeal,而热过程包括 HDA 和 THD。该过程占世界苯产量的 25% 左右。此外,煤焦油也用于生产苯,其中焦油酸用苛性钠去除。最低沸点馏分的蒸馏用于生产苯,然后通过加氢脱烷基化进行纯化 (Schwartz, 2013)。
Sources of Benzene in the Environment
Benzene finds its way to the atmosphere through various processes, both directly and indirectly. It is directed emitted to the environment through exhausts of gasoline vehicles. Industrial processes utilizing or manufacturing benzene also release benzene to the air. They include oil refineries, shoe manufacturers, chemical plants, rubber industry, and gasoline-related plants. Products that also use benzene as an intermediary such as dyes, pesticides, detergents, drugs, and lubricants also emit the benzene vapor to the environment.
In addition, water released from benzene-related industrial processes and practices in the form of effluents and spills drain into water bodies causing contamination. Smoking cigarette and other tobacco products release benzene into the air through the smoke particles. Coke ovens, production of nonferrous metal, coal and ore mining, textile manufacture and processing of wood and wooden products indirectly emit benzene to the environment. The benzene released to the soil may leach through the ground into the underground reservoirs and aquifers; thus, contaminating underground water. The benzene release to the air is in the vapor phase, which dissolves in atmospheric water and rain may wash it from the environment (Schwartz, 2013).
译文:
环境中苯的来源 有机化合物essay代写
苯通过各种过程直接或间接进入大气。它通过汽油车的尾气直接排放到环境中。使用或制造苯的工业过程也会将苯释放到空气中。它们包括炼油厂、制鞋厂、化工厂、橡胶工业和与汽油相关的工厂。也使用苯作为中间体的产品,如染料、杀虫剂、清洁剂、药物和润滑剂,也会将苯蒸气排放到环境中。
此外,与苯相关的工业过程和实践以废水和溢出物的形式释放的水排入水体,造成污染。吸烟和其他烟草产品通过烟雾颗粒将苯释放到空气中。焦炉、有色金属生产、煤炭和矿石开采、纺织品制造以及木材和木制品的加工间接向环境排放苯。释放到土壤中的苯可能通过地面渗入地下水库和含水层;从而污染地下水。释放到空气中的苯呈气相,会溶解在大气中的水中,雨水可能会将其从环境中冲走(Schwartz,2013 年)。
Effects of Contamination with Benzene
Benzene compounds can enter the body through inhaling contaminated air especially in poorly ventilated areas and living near gasoline stations, and consumption of contaminated water or food. Contamination through the skin can also occur when one makes contact with benzene-based products such as gasoline, but this is rare since benzene is volatile. Benzene is thought to cause cancer and other terminal illnesses such as leukemia and other disorders relating to blood cells. Firefighters, printers, steel workers, and lab technicians are the groups most susceptible to contamination with benzene because they work in industries that involve benzene in their production process. In a study conducted, the aforementioned group of workers had higher levels of benzene and the rates of leukemia were frequent in the group.
Various forms of cancer such as such as acute lymphocytic leukemia, chronic lymphocytic leukemia and acute myeloid leukemia were detected in people exposed to high levels of benzene.
Severe anemia and lymphoma were also common in the susceptible group because benzene adversely affects blood cells. Other health effects associated with benzene include drowsiness, confusion, tremors, dizziness, unconsciousness, and headaches. Problems associated with consumption of benzene-contaminated food or water include stomach upset, convulsions, vomiting, and rapid breathing. Benzene also causes low blood cell count leading to weakened immunity and excessive bleeding (American Cancer Society, 2013).
However, the government, through the Occupational Safety & Health Administration (OSHA) controls the levels of exposure to benzene at the work place. The level of benzene in the air should not exceed 1 part per million and a maximum of 5 parts per million in every 15 minutes. The federal agency also requires workers in high-risk zones to have personal protective equipment, for example, respirators, to minimize the level of exposure. Individuals and workers can also limit their subjection to benzene by taking appropriate precautionary measures. Cessation of smoking is one of the ways to limit benzene exposure because cigarette smoke has high levels of benzene. Wearing gas masks while at work and minimizing exposure to products containing benzene such as paints and solvents are also vital in reducing exposure to benzene fumes (Singh, 2004).
译文:
苯污染的影响 有机化合物essay代写
苯化合物可以通过吸入受污染的空气进入人体,尤其是在通风不良的地区和住在加油站附近,以及食用受污染的水或食物。当人们接触汽油等苯类产品时,也会发生皮肤污染,但这种情况很少见,因为苯具有挥发性。苯被认为会导致癌症和其他绝症,如白血病和其他与血细胞有关的疾病。消防员、打印机、钢铁工人和实验室技术人员是最容易受到苯污染的群体,因为他们在生产过程中涉及苯的行业工作。在进行的一项研究中,上述一组工人的苯含量较高,并且该组中的白血病发病率很高。
在接触高浓度苯的人群中检测到各种形式的癌症,例如急性淋巴细胞白血病、慢性淋巴细胞白血病和急性髓细胞白血病。
由于苯会对血细胞产生不利影响,因此易感人群中也很常见严重贫血和淋巴瘤。与苯相关的其他健康影响包括困倦、意识模糊、颤抖、头晕、失去知觉和头痛。与食用受苯污染的食物或水相关的问题包括胃部不适、抽搐、呕吐和呼吸急促。苯还会导致低血细胞计数,导致免疫力下降和出血过多(美国癌症协会,2013 年)。
然而,政府通过职业安全与健康管理局 (OSHA) 控制工作场所接触苯的水平。每 15 分钟,空气中的苯含量不应超过百万分之一,最多为百万分之五。联邦机构还要求高风险地区的工人配备个人防护设备,例如呼吸器,以尽量减少接触水平。个人和工人也可以通过采取适当的预防措施来限制他们接触苯。戒烟是限制苯暴露的方法之一,因为香烟烟雾中的苯含量很高。在工作时戴上防毒面具并尽量减少接触油漆和溶剂等含苯产品对于减少接触苯烟雾也很重要(Singh,2004 年)。
Physical Properties of Benzene
The chemical formula of benzene is C6H6 and a molecular weight of 78.11 g/mol. Its vapor pressure is 100 mm Hg at 26.1 C, and its solubility is 1.8 g/L of water at 25° C. It has a melting point of 5.5°c, a boiling point of 80.5°c and a density of 0.87g cm-3, making it slightly lighter than water. It increases with the increasing molecular mass in the homologous series because it has strong Vander Waal’s forces. Benzene burns with a sooty flame because of the many number of carbon atoms in its structure, and it is highly flammable. It is a stable compound because of the positioning of the double bonds, which gives it resonance and the ability to exist in various forms. Therefore, benzene undergoes only substitution reactions instead of additional reactions with other compounds (Stoker, 2011).
译文:
苯的物理性质
苯的化学式为 C6H6,分子量为 78.11 g/mol。 其蒸气压在26.1℃时为100mmHg,在25℃时溶解度为1.8g/L水。熔点5.5℃,沸点80.5℃,密度0.87g·cm -3,使其比水略轻。 它随着同系中分子质量的增加而增加,因为它具有很强的范德华力。 苯由于其结构中有许多碳原子,因此燃烧时会产生煤烟火焰,并且高度易燃。 由于双键的定位,它是一种稳定的化合物,这使其具有共振和以各种形式存在的能力。 因此,苯只发生取代反应,而不与其他化合物发生额外反应 (Stoker, 2011)。
Chemical Properties
Benzene reacts vigorously with alkyl halides such as alkyl chloride together with the catalysts ethyl aluminum dichloride. It does not react with oxidizing agents like nitric acid. It ignites when in contact with powdered chromic anhydride and other interhalogens such as bromine pentafluoride. It does not have any area of charge either electrical or otherwise in the molecule, making it nonpolar. However, it can react with compounds with an opposite charge such as carbon tetrachloride, alcohol, chloroform, and acetone.
Highly polarized compounds such as bromine react with benzene in substitution reactions to yield brominated benzene. There is the production of hydrochloric acid as a byproduct when benzene reacts with cyanogen halides. Benzene undergoes substitution reactions although it is highly unsaturated, and this characteristic is referred to as aromaticity. Its resonance enables it to undergo electrophilic substitution reactions as opposed to addition reactions in order to maintain its aromatic nature. Two or more benzene rings can join to form various hydrocarbons, which have enhanced reactivity and aromaticity than benzene, for example, naphthalene (Stoker, 2011). A diagram of naphthalene is as shown.
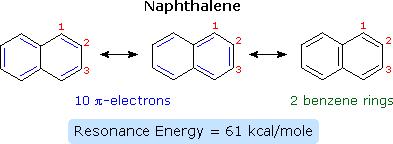
(Stoker, 2011).
译文:
化学性质 有机化合物essay代写
苯与烷基卤如烷基氯以及催化剂乙基二氯化铝剧烈反应。它不与硝酸等氧化剂反应。与粉状铬酸酐和其他卤间化合物(如五氟化溴)接触时会燃烧。它在分子中没有任何带电区域或其他区域,使其非极性。但是,它可以与四氯化碳、酒精、氯仿和丙酮等带相反电荷的化合物发生反应。
高度极化的化合物如溴在取代反应中与苯反应生成溴化苯。当苯与卤化氰反应时,会产生盐酸作为副产物。苯虽然高度不饱和,但会发生取代反应,这种特性被称为芳香性。它的共振使其能够进行亲电取代反应而不是加成反应,以保持其芳香性。两个或多个苯环可以连接形成各种烃,这些烃比苯具有更高的反应性和芳香性,例如萘 (Stoker, 2011)。萘的图表如图所示。(斯托克,2011)。
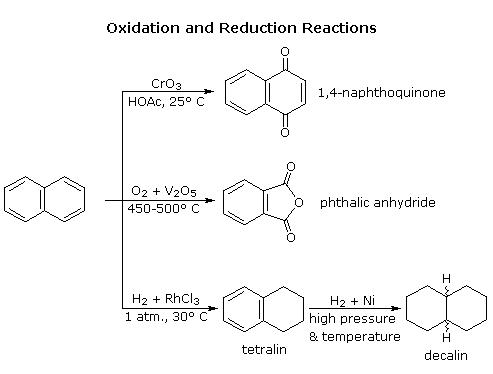
Naphthalene has a higher reactivity than benzene and some of the reduction and oxidation reactions involving naphthalene are elaborated diagrammatically.(Stoker, 2011)
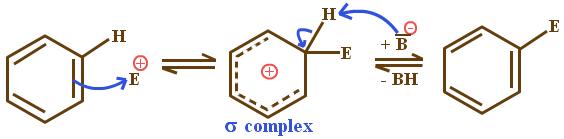
Electrophilic substitution reactions occur in various stages. The first step involves a reaction between co-reagents and catalysts yielding a strong electrophilic species. An interaction of benzene with electrophiles results in the formation of cyclohexadienyl cation, which is referred to as the arenium ion, the σ complex or the Wheland complex. Step 2 is the reaction of the complex with a base to yield a substituted product, a process known as deprotonation.
(Singh, 2004).
The table below shows various electrophilic substitution reactions, conditions for the reactions, the products and by-products.
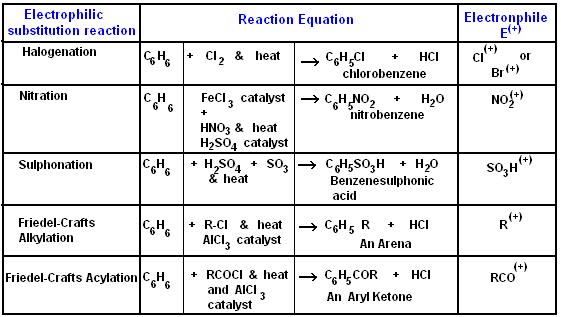
(Singh,2004)
An example of electrophilic substitution reaction is a reaction between bromine or chlorine and benzene to form halobenzene in the presence of aluminium salts of corresponding halogen or Lewis acid.
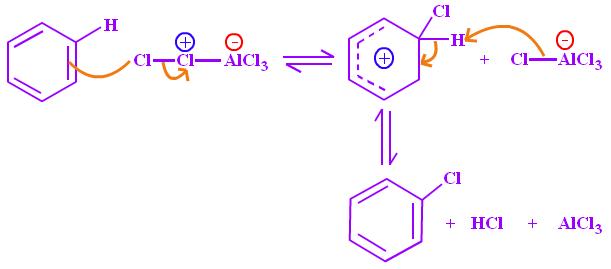
Singh, 2004).
Benzene also undergoes addition reactions such as those involving alkynes and alkenes under special conditions yielding stable additional products. For example, hydrogenation is an additional reaction involving benzene and nickel or palladium catalyst to yield cyclohexane at a temperature of 475-500K.
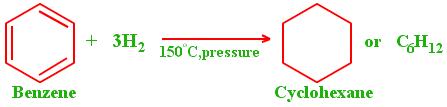
(Singh, 2004).
Halogenation between chlorine and benzene is also an example of an additional reaction, which yields benzene hexachloride, an insecticide, in the presence of sunlight sans a catalyst.
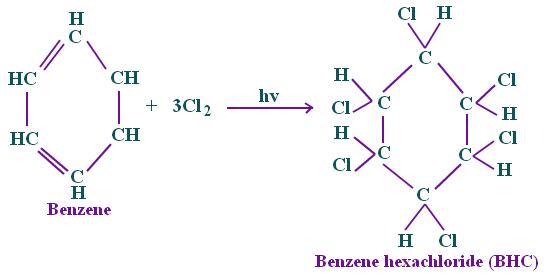
(Stoker, 2011).
The third chemical property of benzene is the ability to yield water and carbon dioxide where it is oxidized through combustion.
2C6H6 + 15O2 → 2CO2 + 6H2O + heat
Oxidation of benzene also occurs in the presence of vanadium pentaoxide catalyst at a temperature of 725 K to yield maleic anhydride.
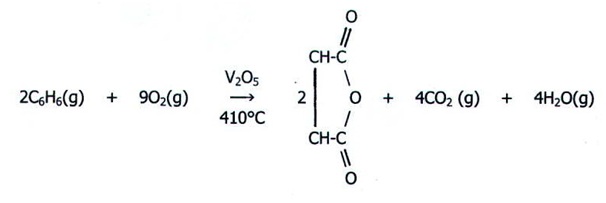
(Singh, 2004).
译文:
萘具有比苯更高的反应性,并且对涉及萘的一些还原和氧化反应进行了图示说明。(斯托克,2011)
亲电取代反应发生在各个阶段。第一步涉及共试剂和催化剂之间的反应,产生强亲电子物质。苯与亲电子试剂的相互作用导致环己二烯基阳离子的形成,称为铪离子、σ 络合物或惠兰络合物。第 2 步是配合物与碱反应生成取代产物,这一过程称为去质子化。 (辛格,2004 年)。
下表显示了各种亲电取代反应、反应条件、产物和副产物。 (辛格,2004)
亲电取代反应的一个例子是溴或氯与苯在相应卤素或路易斯酸的铝盐存在下反应生成卤苯。(Singh,2004)。
苯也会在特殊条件下发生加成反应,例如涉及炔烃和烯烃的加成反应,产生稳定的附加产物。例如,氢化是涉及苯和镍或钯催化剂的附加反应,在 475-500K 的温度下产生环己烷。 (辛格,2004 年)。
氯和苯之间的卤化也是附加反应的一个例子,在没有催化剂的情况下,在阳光下产生六氯化苯,一种杀虫剂。 (斯托克,2011 年)。
苯的第三个化学性质是通过燃烧产生水和二氧化碳的能力。
2C6H6 + 15O2 → 2CO2 + 6H2O + 热量
在五氧化二钒催化剂存在下,在 725 K 的温度下,苯也发生氧化,生成马来酸酐。(Singh,2004 年)。
Uses of Benzene
Benzene is used as a key component in various products such as adhesives, paints, rubber, detergents, and coatings. It is also important in dry cleaning, lithography and printing, graphic designing where ink is needed, rectification, extraction, and in the shoe and tire industries. It is used to attach soles of shoes, clean the components of the fuel system, brakes, and hydraulic systems, and reducing knocking of engines in gasoline vehicles. In addition, benzene is an essential intermediary in the synthesis of nylon, phenolic resins, synthetic rubber, polyester resins, detergents, aniline, chlorobenzenes and polystyrene plastics (Schwartz, 2013).有机化合物essay代写
It is also necessary during the production of insecticides, explosives, lacquers, drugs, aniline, varnishes, plastics and dyes. Benzene also serves as an essential solvent for inks, waxes, oils and fats especially in the extraction of oil from nuts and seeds. Benzenoids are mainly incorporated in the synthesis of polymers and resins (American Cancer Society, 2013). The table below shows various benzoids and the products derived from them.
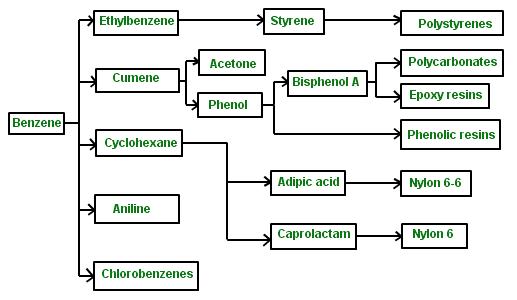
(Schwartz, 2013)
译文:
苯的用途
苯用作各种产品的关键成分,例如粘合剂、油漆、橡胶、清洁剂和涂料。它在干洗、平版印刷和印刷、需要墨水的图形设计、整改、提取以及制鞋和轮胎行业中也很重要。用于固定鞋底,清洁燃油系统、刹车和液压系统的部件,以及减少汽油车发动机的爆震。此外,苯是尼龙、酚醛树脂、合成橡胶、聚酯树脂、洗涤剂、苯胺、氯苯和聚苯乙烯塑料合成过程中必不可少的中间体(Schwartz,2013 年)。
在杀虫剂、炸药、漆、药物、苯胺、清漆、塑料和染料的生产过程中也是必要的。苯还用作油墨、蜡、油和脂肪的重要溶剂,尤其是在从坚果和种子中提取油时。苯类化合物主要用于聚合物和树脂的合成(美国癌症协会,2013 年)。下表显示了各种苯甲类化合物及其衍生产品。 (施瓦茨,2013 年)
Conclusion
Benzene is a commonly used organic compound in industries and other institutions globally. It has numerous industrial uses including being a significant constituent of gasoline, a solvent for many processes, a cleaner for major parts of machinery and equipment, and manufacturing of plastics, polyester resins and polymers. Benzene undergoes mainly substitution reactions, but there are also additional reactions with alkenes and alkynes under special conditions. The substitution reactions, which include sulphonation, nitration, and halogenation, are essential in maintaining the sweet smell (Singh, 2004). Subjection to high benzene levels has adverse health effects because the compound is associated with leukemia and other blood disorders. Therefore, the government has put regulatory measures to help minimize exposure to benzene at work places
译文:
结论 有机化合物essay代写
苯是全球工业和其他机构常用的有机化合物。 它有许多工业用途,包括作为汽油的重要成分、许多工艺的溶剂、机械和设备主要部件的清洁剂以及塑料、聚酯树脂和聚合物的制造。 苯主要发生取代反应,但在特殊条件下也会与烯烃和炔烃发生额外的反应。 包括磺化、硝化和卤化在内的取代反应对于保持甜味至关重要 (Singh, 2004)。 苯含量过高会对健康产生不利影响,因为该化合物与白血病和其他血液疾病有关。 因此,政府已采取监管措施,以帮助尽量减少工作场所接触苯的情况。
References 有机化合物essay代写
American Cancer Society. (2013). Benzene. Retrieved on 8 Dec. 2013 from http://www.cancer.org/cancer/cancercauses/othercarcinogens/intheworkplace/benzene
Schwartz, A. (2013). Uses of benzene: Unsafe products cause cancer and leukemia. Anapol Schwartz: Personal Injury Lawyers. Retrieved on 8 Dec. 2013 from http://www.anapolschwartz.com/practices/benzene/benzene-uses.asp
Singh. (2004). Advanced organic chemistry: Reactions and mechanisms. New Delhi: Pearson Education India.
Stoker, S.H. (2011). General, organic, and biological chemistry, 6th ed. Boston MA: Cengage Learning.

其他代写:代写CS C++代写 java代写 matlab代写 web代写 澳大利亚代写 作业代写 物理代写 数学代写 考试助攻 paper代写 r代写 金融经济统计代写 python代写

