Chemistry 231.3 – Inorganic Chemistry I 2021-2022 Term 1
无机化学作业代写 Note that the coordination numbers around cations and anions will not necessarily be the same for non 1:1 stoichiometries.
Assignment #1: Module 1 无机化学作业代写
Due Friday, September 24th at 8:30 am (i.e. at beginning of synchronous lecture) Total Marks: 30
- (a) Gold packs in a fcc array, with a unit cell dimension of 4.08 Å. (4marks)
(i)What is the atomic radius of a gold atom in this fcclattice?
(ii)What is the estimated density of gold based on this unit cell, and how does it compare to the actual density of gold? (You’ll need to look up atomic mass of Au to do thisquestion)
(b) Metallic potassium (K) adopts a bcc structure with a density of 856 kg/m3. (4 marks) 无机化学作业代写
(i)Work out the length of the unit cell using this information.
(ii)Would you expect the unit cell dimensions for bcc rubidium to be smaller or greater than that of potassium?Why?
2.Stainless steel is an alloy made from iron mixed with carbon, nickel, and chromium (and sometimes other elements as well). Detail what positions the dopant atoms would have in the bcc Fe structure for each of the dopant elements above (Hint: they don’t all go in the same locations!). (3marks)

3.Answer the following questions with reference to the zinc blende, rock salt, and rutile structure types: (6 marks) 无机化学作业代写
(a)What type of packing arrangement is seen in each of the three structuresabove?
(b)Indicate coordination numbers for all atoms for all three structure types. Note that the coordination numbers around cations and anions will not necessarily be the same for non 1:1 stoichiometries.
- In structure composed of ccp spheres A, all the tetrahedral holes and all the octahedral holes are occupied by atoms C. (5 marks)
a)Draw a “layer sequence” structure of this compound with the composition AxCy.Do not forget to label all layers and spheres. 无机化学作业代写
b)Determine the stoichiometry of this solid. (i.e. what are x and y in AxCy). Indicate your logic.
5.The CdCl2 structure type is related to the CdI2 structure type, except the Cl anions are packedin a fcc lattice, and ½ of the octahedral holes are filled with Cd cations.
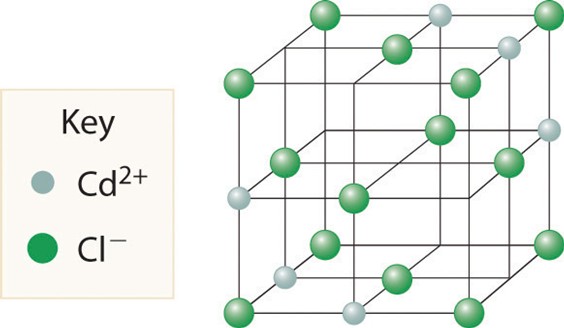
Shown above is an attempted structure of CdCl2 that I found on the internet. Add up the number of cations and anions in the unit cell, and show that the stoichiometry of this compound is NOT CdCl2. Show your work. What is the actual stoichiometry of the shown unit cell? (4 marks)
- (a) What would be the expected coordination environments around the anions and cations inMgO, MgSe, and BkO2 (Bk= berkelium), given the following average atomic radii for 6- coordinate ions: (3 marks) 无机化学作业代写
Mg2+ 0.72 Å O2- 1.40 Å
Bk4+ 0.63 Å Se2- 1.98 Å
(b) What are the actual anion packing arrangements of MgO, MgSe, and BkO2? Are any of them different than expected? Note: you’ll have to do a search for these structures online to find this information. (1 mark)

更多代写:计算机代修网课 多伦多Online Quiz代考 多伦多essay代写 多伦多网课essay代写 法律law论文代写 财务管理论文代写

