CHEM 001-02
Exam 3
(100 pts)
化学代考 Based on molecular mass and dipole moment of the five compounds in the table below, which should have the highest boilingpoint?
This is a closed-book examination lasting 50 minutes. You may not use any outside information other than the supplement that was provided. You may use your calculator.
In order to gain partial credit, answers must be presented clearly. Unless otherwise stated, please provide your reasoning or show your calculations to receive full credit for a problem. 化学代考
My signature signifies that I have neither given nor received help on this exam.
Honor Pledge (please sign):
| Section | Points | Score |
| I | 10 | |
| II | 12 | |
| III | 20 | |
| IV | 8 | |
| V | 12 | |
| VI | 8 | |
| VII | 6 | |
| VIII | 12 | |
| IX | 12 | |
| TOTAL | 100 |
Intermolecular Forces.

(10 pts) I. Multiple Choice. Circle the best answer. 化学代考
1.Which of the following molecules would deviate from ideal gas behavior the most at 298K?
A)Ne
B)O2
C)H2O
D)CO
E)H2S
2.Of the followingsubstances, only has London dispersion forces as the only intermolecular forces:
A)CH3OH
B)NH3
C)H2S
D)Kr
E)HCl
3.Based on molecular mass and dipole moment of the five compounds in the table below, which should have the highest boilingpoint?
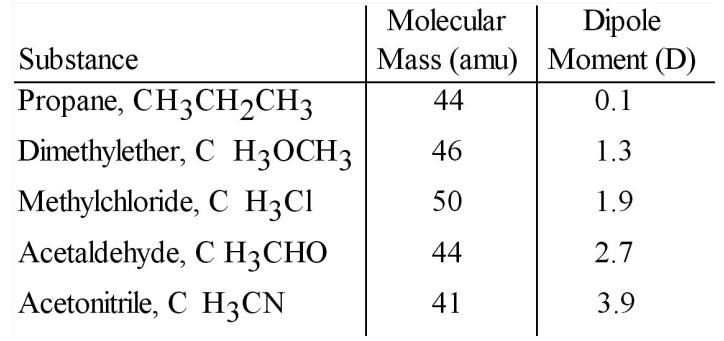
A)CH3CH2CH3
B)CH3OCH3
C)CH3Cl
D)CH3CHO
E)CH3CN
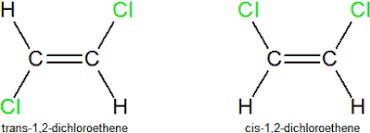
4.Which has a higher boiling point, trans-dichloroethene higher orcis-dichloroethene?
Solid-State
(12 pts) II. Multiple Choice: circle the best answer.
1.Crystalline solids differ from amorphous solids in that crystallinesolids have .
A)appreciable intermolecular attractiveforces
B)a long-range repeating pattern of atoms, molecules, orions
C)atoms, molecules, or ions that are closetogether 化学代考
D)much larger atoms, molecules, orions
E)no orderlystructure
2.If a cubic unit cell of an ionic compound has A cations at the corners and the face centers and X anions in the centers of the edges, what is the empirical formula of thecompound?
A)AX
B)A4X3
C)AX2
D)A2X3
E)A2X
3.How many calcium and fluorine ions are there in the unit cell shownbelow?
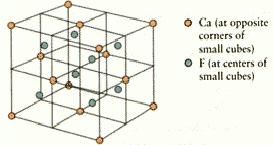
A)4 Ca2+ and 2F-
B)4 Ca2+ and 8F-
C)2 Ca2+ and 4F-
D)2 Ca2+ and 8F-
E)4 Ca2+ and 4F-
(20 pts) III.
Gold crystallizes in a face-centered cubic unit cell with an edge length of 407 pm.
(a)What is the coordination number ofgold?
(b)What is the total number of gold atoms that lie within the unitcell?
(c)What is the atomic radius ofgold? 化学代考
(d)What is the density ofgold?
(e)What percentage of the unit cell is occupied if each atom is treated as a hardsphere?
(8 pts) IV. 化学代考
(a) Lithium oxide can be described as a face centered cubic array of oxide ions with lithium ions in all of the tetrahedral holes. What it the formula of lithium oxide?
(b) The length of the edge of a unit cell of lithium oxide is 461 pm. What is the approximate Li- O bond distance?
Thermodynamics: The First Law, Internal Energy, Heat, Work, Enthalpy, Phase Changes (12 pts) V. Multiple Choice: circle the best answer.
1.Which one of the following conditions would always result in an increase in the internal energy of asystem?
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of the above is correct.
2.Of the following, which one is a statefunction? 化学代考
A)enthalpy
B)heat
C)work
D)q
E)none of theabove
3.What is the total motional contribution to the molar internal energy of gaseousHCN?
A)3RT
B)5RT
C)5RT
D)RT
E)5RT
(8 pts) VI. Under constant volume conditions, the heat of combustion of benzoic acid (C6H5COOH) is 26.38 kJ/g. A 2.76 g sample of benzoic acid is burned in a bomb calorimeter. The temperature of the calorimeter increases from 21.6 to 29.9 °C.
(a)Write a balanced equation for the combustion of benzoic acid.
(b)What is the total heat capacity of thecalorimeter? 化学代考
(c)Suppose that in changing samples a portion of the water in the calorimeter were lost. In what way, if any, would this change the heat capacity of the calorimeter.
(6 pts) VII. (a) Calculate the ΔU of a system that releases 12.4 J of heat and does 4.2 J of work on the surroundings.
(b) A sample of an ideal gas in a cylinder of volume 3.77 L at 298 K and 2.01 atm expands to 7.41 L isothermally and reversibly. Calculate the work.
Enthalpies of Reactions 化学代考
(12 pts) VIII. Calculate DH for the reaction:
Na2O(s) + SO3(g) à Na2SO4 (g)
Given the following:
Na(s) + H2O(l) à NaOH (s) + ½ H2(g) DH = -146 kJ Na2SO4(s) + H2O(l) à 2 NaOH(s) + SO3(g) DH = +418 kJ 2 Na2O(s) + 2 H2(g) à 4 Na(s) + 2 H2O(l) DH = +259 kJ
(12 pts) IX. Consider an ionic compound, MX, composed of generic metal M and generic, gaseous halogen X. 化学代考
- The enthalpy of formation of MX is ΔHf = −445kJ/mol.
- The enthalpy of sublimation of M is ΔHsub = 141kJ/mol.
- The ionization energy of M is IE = 481kJ/mol.
- The electron affinity of X is ΔHEA = −313kJ/mol.
- The bond energy of X2 is BE = 201kJ/mol.
Determine the lattice energy of MX.

